What is methane?
Methane (CH4) is one of the most powerful, abundant and surprising greenhouse gases. It is defined in chemistry as a colorless, odorless flammable gas which is the main constituent of natural gas. It is the simplest member of the alkane series of hydrocarbons and is the most commonly found gas. It is a chemical compound that shows up nearly everywhere on the planet. Why and where it shows up is often a mystery. In many ways it is an unknown. The story of methane is a complex and fascinating tale with many current controversies.
What forms does methane take?
Methane can be a gas or a solid, generally this is described as atmospheric or in clathrates. According to Wikipedia, at room temperature and standard pressure, methane is most commonly found in the atmosphere as a gas. The relative abundance of methane on Earth makes it an attractive fuel, although capturing and storing it poses challenges due to its gaseous state under normal conditions for temperature and pressure.
Solid methane exists in several modifications known as clathrates. These substances crystallize in a cubic system. Methane clathrate, also called methane hydrate, hydromethane, methane ice, fire ice, natural gas hydrate, or gas hydrate, is a solid clathrate compound (more specifically, a clathrate hydrate) in which a large amount of methane is trapped within a crystal structure of water, forming a solid similar to ice.
Originally thought to occur only in the outer regions of the Solar System, where temperatures are low and water ice is common, significant deposits of methane clathrate have been found under sediments on the ocean floors of the Earth. The clathrate primer provided below will discuss this unique formation further.
Methanogenesis
Methane is mainly produced by methanogenesis that is both geological and biological. This multi-step process is used by microorganisms as an energy source. According to Wikipedia the two main routes for geological methane generation are (i) organic (thermogenic/biogenic) and (ii) inorganic (abiotic, meaning non-living). Both ways can involve microorganisms but may also occur inorganically.
Methane and carbon dioxide are made when permafrost thaws as dead animal and plant remains are decomposed. However, methane is only formed if no oxygen is available. According to a recent study, “until now, it was assumed that larger amounts of greenhouse gases are formed when the ground was dry and well aerated—when oxygen was available. Christian Knoblauch and his colleagues have now demonstrated that water-saturated permafrost soils without oxygen can be twice as harmful to the climate as dry soils—which means the role of methane has been greatly underestimated.”
Why does methane matter?
Methane matters in the climate system because, even though it is relatively short-lived, it is one of a number of trace gases that act to trap outgoing radiation leading to an overall warming of the climate system. Its effects are far reaching and powerful. Methane impacts climate sensitivity through a mechanism known as radiative forcing. Methane poses a powerful threat to the climate system. Methane also matters for other reasons to do with air quality and human health impacts.
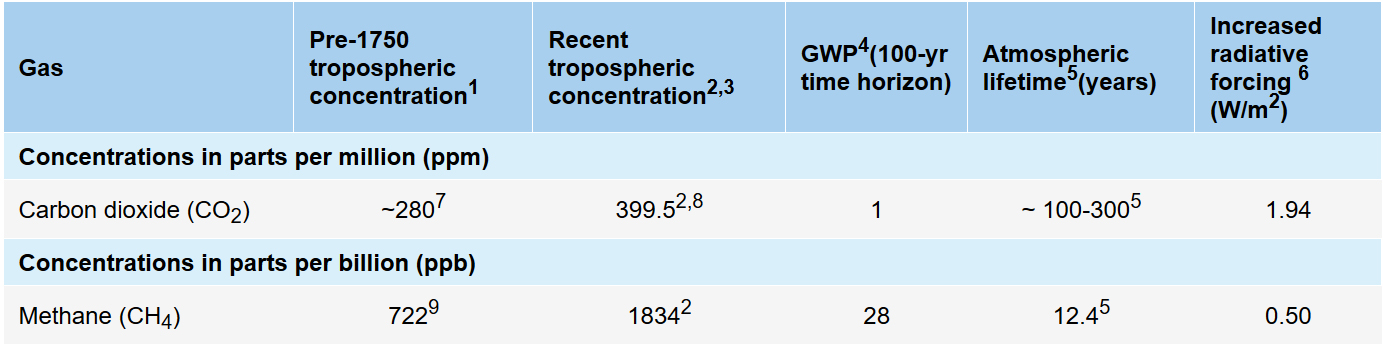
It is known to have a greater greenhouse gas (GHG) warming factor than CO2, a potential of 34 times that of CO2 over 100 years according to the latest IPCC AR5 Assessment Report. These findings were included in the report which also states that “the 100-year global warming potential (GWP) of methane according to the study is 34, the 20-year GWP is 86, the 10-year is 99, and the 5-year GWP was found to be 115.” Recently shocking reports have emerged showing global atmospheric or gaseous methane literally going off the charts (video). In December 2018, the Copernicus Atmosphere Monitoring Service (CAMS) changed its methane scale to address this. The CAMS Global Methane Forecast provides continuous data and information on atmospheric composition in an operational mode.
According Rupple et al, the present-day concentration of CH4 has risen by ~150% since pre-industrial times, compared to only ~40% for CO2 (IPCC). Rising atmospheric CH4 concentrations lead to more rapid depletion of the hydroxyl radicals (OH) needed for oxidation, longer CH4 residence times, and thus increased CH4-induced warming (Lelieveld et al. 1998).
Since 2007, many studies are discussing a sharp rise in methane. A survey of methane isotope abundance (14C, 13C, 2H) from five nearshore marine basins that reveals unusual radiocarbon levels in subsurface waters. Additionally, the isotopic evidence presented here suggests that the methane rise was dominated by significant increases in methane emissions from anthropogenic and other sources that will be discussed below.
Methane by the Numbers
Let’s look at the numbers and trends for methane concentrations from all sources. You will notice that at times these numbers vary by study and that different researchers use slightly different calculations and methods but their results are very close. Many of these estimates here also entail approximations.
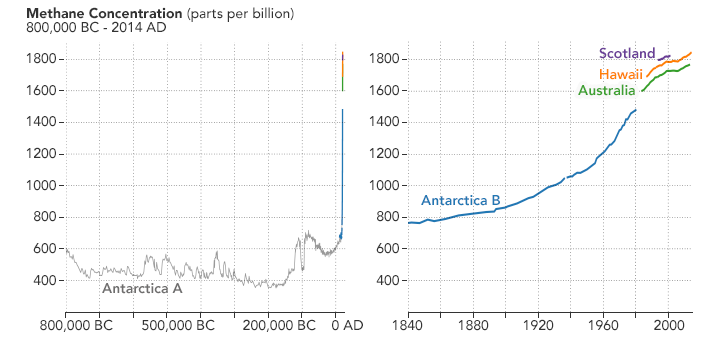
Global methane levels have been going up steadily. The following chart below, based on data collected by the National Oceanic and Atmospheric Administration (NOAA), shows variations in the rate of increase in the concentration of methane in the atmosphere between 1984 and 2014. Very strong atmospheric methane growth in the years 2014‐2017 also gives cause to watch this trend closely.
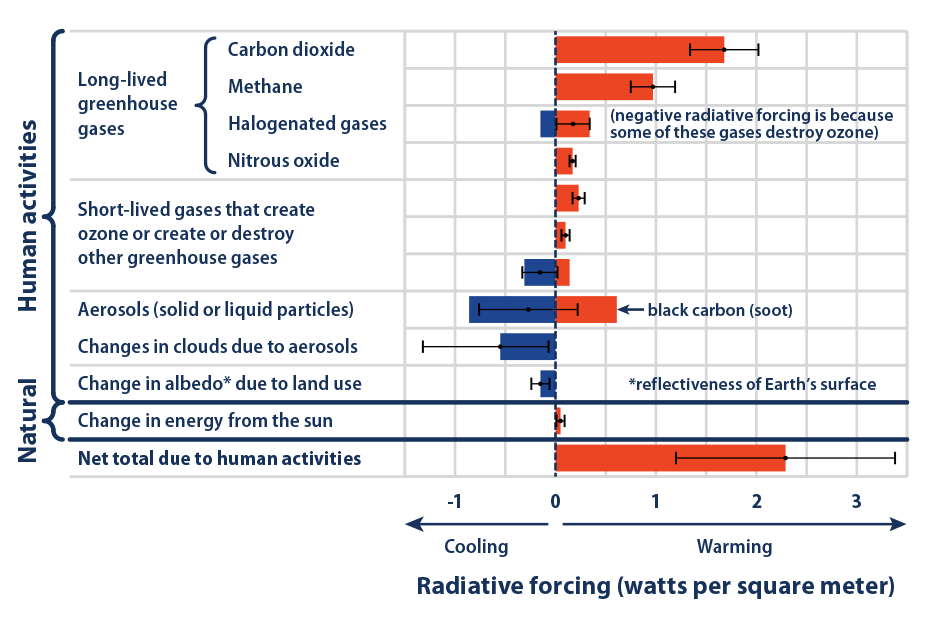
The following chart shows the radiative forcing contribution relative to other GHG sources at present. The Global Warming Potential (GWP) of CH4 is estimated to be 28–36 over 100 years. That is, over a 100-year period, it traps 28 times more heat per mass unit than CO2 and ~32 times the effect when accounting for aerosol interactions. About 20-25% of the man-made global warming we’re experiencing is caused by methane emissions. The climate sensitivity to CH4 is significant:
The concentration of methane in the atmosphere is now at 1.83 ppm. Concentrations of methane in the atmosphere are about 220 times lower than carbon dioxide now at over 400 ppm. Both numbers are much higher than at any time during the last 650,000 – 800,000 years.
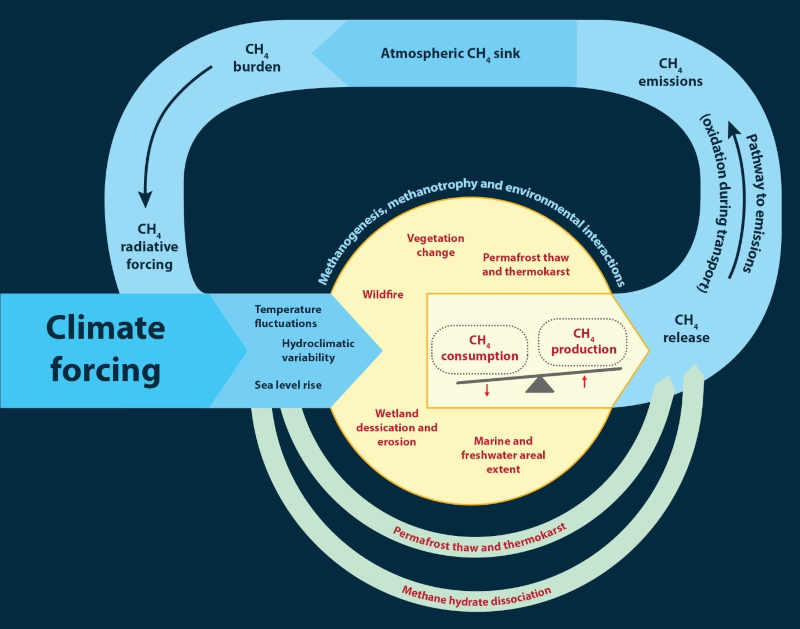
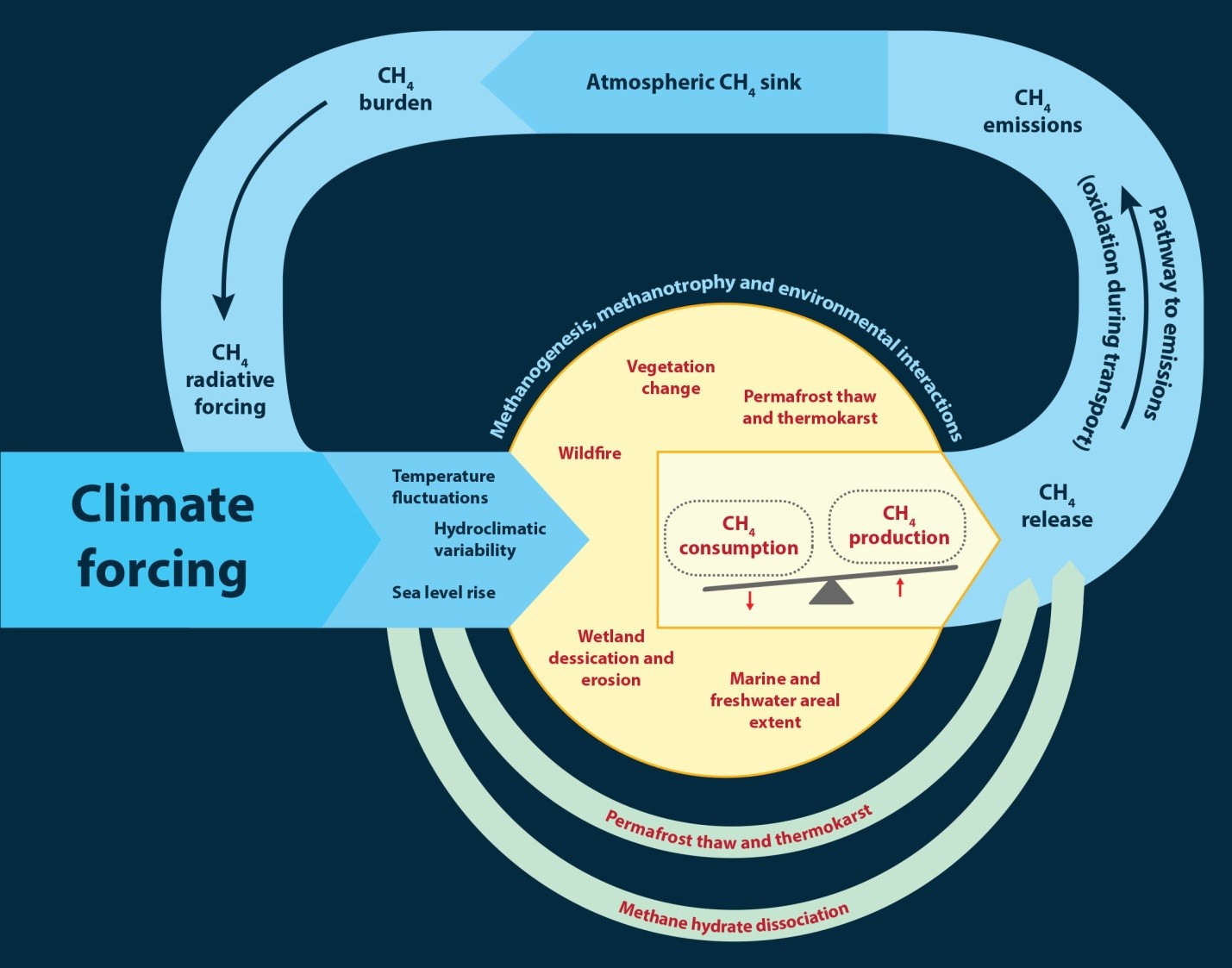
Methane is a very reactive gas compared to CO2. It was responsible for 60% of the equivalent radiative forcing caused by carbon dioxide since the onset of the Industrial Revolution. Methane is a powerful contributor to the greenhouse gas climate forcing. The following illustration depicts how this feedback system works:
CH4 emitted today lasts about a decade on average, which is much less time than CO2. CH4 breaks down much more quickly than CO2 and is found at much lower levels in the atmosphere. Simply put, methane has a large effect, but for a relatively brief period, having an estimated lifetime of about 10 – 20 years in the atmosphere, whereas carbon dioxide has a small effect for a long period, having an estimated lifetime of over 100 years. CH4 is initially far more devastating than CO2 to the climate because of how effectively it absorbs heat.
Even though CH4 is only around for maybe a decade or so, during that time it also absorbs much more energy than CO2. The net effect of the shorter lifetime and higher energy absorption is reflected in the GWP. The CH4 GWP also accounts for some indirect effects, such as the fact that CH4 is a precursor to ozone, and ozone is itself a GHG. Methane in the atmosphere can also affect the abundance of other greenhouse gases, such as ozone (O3), water vapor (H2O), and carbon dioxide. It traps more heat this way. Methane is considered the third most potent GHG after CO2 and H2O.
Therefore in just a couple decades, methane warms the planet by 86 times as much as CO2, according to the IPCC. But policymakers typically ignore methane’s warming potential over 10 – 20 years (GWP20) when assembling a nation’s emissions inventory. According to this study “net-zero cost emission reductions can lead to a declining atmospheric burden, but can take three decades to stabilize.” This is referred to as the climate lag time for methane as well as other GHGs. This also means that even if we stop all emissions right now, we are still facing disastrous feedback effects as the consequences of our actions today for several decades to come.
If you count methane in terms of atoms of carbon, it is clear that methane emissions add more carbons to an increasingly carbon saturated atmosphere. The following charts distinguishes breakout factors in radiative climate forcing for comparison, but we need to keep in mind that these compounds all work together in complicated synergies that are not reflected here:It is important to note that Arctic methane is a small percentage of the total global methane budget. According to David Archer, climate scientist, compared to natural wetland and anthropogenic sources, Arctic methane represents only a very tiny fraction:
To sum up, scientists attribute about one-sixth of recent global warming to all methane emissions; what methane lacks in volume it makes up for in potency. Over a 20-year period, one ton of methane has a global warming potential that is 84 to 87 times greater than carbon dioxide. “That means the climate effects of methane are front-loaded,” explains Drew Shindell, a climate scientist at Duke University.
Methane Sources: Anthropogenic Versus Natural
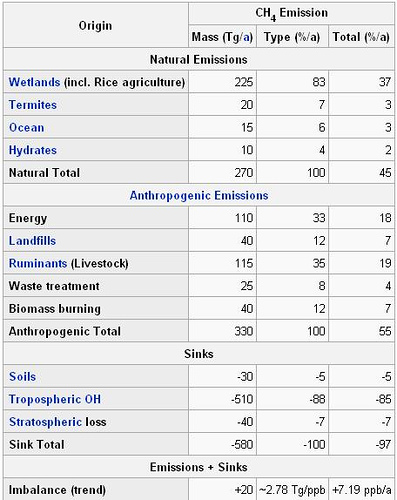
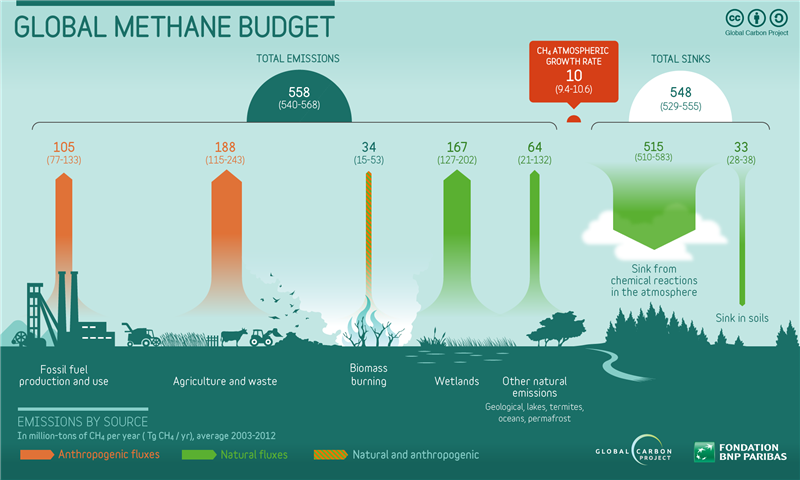
As we have seen methane is a greenhouse gas with many sources, both man-made and natural. Let’s look more closely at the sources of methane and how they interact. Determining the methane source can help us understand how it will impact the climate system. The following chart provides emission origins for methane and rates by mass:
Some of the most prevalent sources of methane (about 60%) come from human activity. According to Dean et al. [2018], “anthropogenic sources are slightly larger emitters of methane to the atmosphere compared to natural sources.” This includes emissions from fossil fuel production and coal use. Methane is also a key by-product of the rapidly rising global extraction and processing of natural gas. The oil and gas industry is responsible for extraction leaks, pipeline leaks, and so forth.
According to Robert Scribbler, climate science blogger, “global methane is again hitting a rapid rate of rise. Though the Earth System appears to be providing some ominous rumblings that feedbacks may be on the way, the present spike is likely primarily due to increased fossil-fuel extraction activity, particularly due to fracking.” Fracking is another controversial topic, but many researchers are discussing how it has become a primary source of human-caused methane emissions. Of course, this is refuted by the industry itself.
Methane also comes from fires and coal mines. Additionally, methane is produced by cattle, and also comes from decaying vegetation. Top sources of methane come from the digestive process of livestock and from landfills, and peat bogs which emit it as waste decomposes. Some are now saying the emissions from the agricultural industry are actually topping fossil fuel outputs. This is a significant concern, but it is often overlooked and/or downplayed.
On the other hand, natural sources of methane dominated by wetlands emissions are responsible for about 40% of global the methane budget. Contributing approximately 200 Tg/yr of methane to the atmosphere per year; wetlands are the largest natural source of atmospheric methane in the world, and therefore remain a major area of concern with respect to climate change. Fermentation is a process used by certain kinds of microorganisms to break down essential nutrients. In a process called acetoclastic methanogenesis, microorganisms from the classification domain archaea produce methane by fermenting acetate and H2-CO2 into methane and carbon dioxide.
What’s the big deal about Arctic methane?
The Arctic region itself is one of the many natural sources of the greenhouse gas methane. It is important to note right off the bat that Arctic methane sources represent less than 1% of the current methane budget at .0003 GTC/yr according to experts like Dr. David Archer. Concerns regarding methane in the Arctic, center around the vast inventory of stored methane hydrates in clahtrates, a subject of much controversy. The following video introduces this topic very briefly:
Recently there have been several fluctuations in the Arctic that have gotten the attention of researchers. In a 2010 study, atmospheric methane levels in the Arctic were measured at 1850 nmol/mol. This level is over twice as high as at any time in the last 400,000 years. Historic methane concentrations in the world’s atmosphere have ranged between 300 and 400 nmol/mol during glacial periods commonly known as ice ages, and between 600 and 700 nmol/mol during the warm interglacial periods.
There are many more such instances being logged. Additionally, increased reports of thermokarst or thaw lakes, ebullition or bubble lakes, polynyas, pingos, craters, seeps, and more are being reported as potential sources of methane leaks from thawing permafrost at shallow depths. This paper describes many of these methane transport mechanisms. Scientists are still trying to determine the reasons for these increases. The fear is that more of these reportedly nascent hydrates are releasing their compressed gas reserves than previously thought possible. Additionally, the latest research contends that important details about these increases are not making it into the current climate change data modeling.
Clathrate Primer
The Arctic contains vast quantities of sequestered or trapped methane hydrates, also known as flammable ice, concentrated in clathrates. These can be found in the great frozen regions of the cryosphere, primarily in the marine seabed sediment and permafrost discussed further on the SW Methane Emergency Wiki. No one knows for certain how much methane hydrate is stored in the Arctic. Estimates put the total amount of clathrates in the Arctic at anywhere from 5,000-18,000 GTC.
According to Rupple et al, “globally, an estimated 99% of gas hydrates occurs in the sediments of marine continental margins at saturations as high as 20% to 80% in some lithologies; the remaining 1% is mostly associated with sediments in and beneath areas of high-latitude, continuous permafrost (McIver 1981, Collett et al. 2009).” Other estimates are that it’s 95% in marine sediment and 5% in the permafrost. Estimates do vary some from study to study.

Arctic methane clathrates found in both permafrost and marine sediment can potentially degrade on warming. Among the concerns for sources of sudden release there is the permafrost itself, as well as the sequestered seabed hydrates. There is also the subsea permafrost. Subsea permafrost occurs beneath the seabed and exists in the continental shelves of the polar regions.
Ancient Greenhouse Gases CO2, CH4, and More
For centuries, a massive store of carbon has been locked underground in frozen soils that make up the cryosphere. As Earth’s climate continues to warm, that carbon is escaping into the atmosphere, the result of microbes waking up and digesting once-frozen organic materials – and it’s not just methane, but even more carbon dioxide that could be released.
Global warming and especially the warming oceans are beginning to liberate even more methane and carbon dioxide from existing clathrate stores, and from methanogenesis in rotting biomass generating powerful feedback loops. Large quantities of methane are also stored in the Arctic in natural gas deposits. Other natural sources of methane include submarine taliks, river transport, ice complex retreat, submarine permafrost, permafrost melt at higher latitudes and clathrate dissolution on sea shelves, deep sea, land and more.
Katey Walter Anthony at the University of Alaska, Fairbanks, who led the project that was part of NASA’s Arctic-Boreal Vulnerability Experiment (ABoVE) said that “these ancient greenhouse gases, produced from microbes chewing through ancient carbon stored in the soil, range from 2,000 to 43,000 years old (…) But by the middle to end of the century the permafrost-carbon feedback should be about equivalent to the second strongest anthropogenic source of greenhouse gases, which is land use change,” she said.
In the following video Dr. Miriam Kastner, geochemist, provides a full lecture on methane hydrates, also known as methane clathrates or gas hydrates:
Methane clathrates are common constituents of the shallow marine geosphere and they occur in deep sedimentary structures and form outcrops on the ocean floor. Methane hydrates are believed to form by the precipitation or crystallization of methane migrating from deep along geological faults. Precipitation occurs when the methane comes in contact with water within the sea bed subject to temperature and pressure.
In 2008, research on Antarctic Vostok and EPICA Dome C ice cores revealed that methane clathrates were also present in deep Antarctic ice cores and record a history of atmospheric methane concentrations, dating to 800,000 years ago.. The ice-core methane clathrate record is a primary source of data for global warming research, along with oxygen and carbon dioxide.
According Rupple et al, methane hydrate is an ice-like substance formed when CH4 and water combine at low temperature (up to ~25ºC) and moderate pressure (greater than 30-50 Atmospheres, which corresponds to combined water and sediment depths of 300 to 500 m). Nominally, methane hydrate concentrates CH4 by ~164 times on a volumetric basis compared to gas at standard pressure and temperature. Warming a small volume of gas hydrate could thus liberate large volumes of gas. In cubic meters, 1m3 in clathrate equals 180m3 (of dissociated compressed gas). This is a frightening and powerful hidden potential.
If methane hydrate is underground in the stability zones, a zone and depth of the marine environment at which methane clathrates naturally exist in the Earth’s crust, it can last for millennia. Once it is released or dissociated into the atmosphere, it will break down in anywhere from nine to 20 years as it is exposed to oxygen and water vapor.
Rupple et al have discussed concern about the long-term stability of global gas hydrate deposits. This is rooted in the potential impact that a large CH4 release might have on the global climate. Also, methane that dissolves in the water column is often oxidized to carbon dioxide through microbial processes further acidifying the ocean. In recent models, the longer-lived CO2 oxidation product (Archer et al. 2009), not the CH4 itself (e.g., Harvey & Huang 1992), is credited with causing most of the excess atmospheric warming that would follow large-scale dissociation of methane hydrates as proposed by some emerging and controversial theories.
What is gas hydrate dissociation?
This study discusses some key factors involved in hydrate dissociation like geological processes, exposure of the hydrates to water, oil drilling, warming, depressurization, and so on. The following diagram also illustrates the structure of methane clathrates and some potential pathways for their release:
Researchers are reporting that gas hydrate in the Arctic is already breaking down at some locations even now, mostly associated with subsea permafrost on Arctic Ocean shelves and with hydrate at the upper limit of its stability on temperate-latitude upper continental slopes affected by warming ocean waters.
The following illustration depicts gas hydrate stability zones:
According to scientists, Arctic methane is already dissociating in seeps, thermokarst or thaw lakes, and bubble plumes escaping from subsea as well as on land permafrost. It is very difficult to determine how much hydrate is already dissociating compared to other sources.
It is also known that methane spontaneously dissociates at the edge of the stability zones and this is not necessarily related to climate change. The methane hydrate stability zone in the Arctic is at depths lower than ~100m to over 1220m below the surface, and it is thought that it would take a very long time to thaw out, on the order of at least hundreds if not thousands of years.
According to Rupple et al, “against the backdrop of a well-mixed atmosphere, strong annual fluctuations, and a significant increase in CH4 concentrations over the 20th century, detecting a CH4 signal that is directly attributable to dissociating gas hydrates may always remain challenging.”
Hydrates and Feedbacks
Because of polar amplification, which means that the Arctic warms at least twice the rate of the rest of the planet, the Arctic is extremely susceptible to perturbations of climate feedbacks. Feedbacks like decreasing albedo because of loss of sea ice (video) and ocean temperatures matter when it comes to increased permafrost thaw, and thus pathways for methane hydrate release. Multi-year ice in the Arctic is nearly gone. The Arctic has declined by over 60% in the last couple of decades and it is also said to be half of its previous thickness according to eminent climate scientist and polar explorer, Peter Wadhams.
Wadhams further states that “If we look at the Arctic death spiral it is clear, in fact it is blindingly obvious, that the summer Arctic sea ice does not have long to live. The downward trend will bring the summer months to zero ice cover….” Wadhams has said that we will see a blue ocean event sooner than expected. These are dramatic issues heralding cause for a crisis response, though none has yet arrived.
The well-known paper, Trajectories of the Earth System in the Anthropocene, presents the big picture stating, “these feedback processes include permafrost thawing, decomposition of ocean methane hydrates, increased marine bacterial respiration, and loss of polar ice sheets accompanied by a rise in sea levels and potential amplification of temperature rise through changes in ocean circulation.”
Robert Scribbler writes “a report, explaining ocean warming, provides some pretty hard evidence that the oceans are on the move toward a much more harmful global climate state. The study, which has rightly received a great deal of media attention, issues a ‘shot across the bow’ warning to pretty much everyone living today. And it finds serious impacts to the ocean and linked climate systems due to a very rapid human-forced global warming. This has further implications for thawing the polar ice faster, which will release more methane.
There are many more complicated and significant feedbacks being discovered almost daily now. Scarier still, the feedbacks we know of are already triggering unpredictable events beyond the control of human agency, like the 3 feet of sea level rise already expected by 2100 even if we stop emissions on a dime today. Some scientists, like Dr. Harold Wanless, sea level rise expert, have said we are actually in for as much as 130 feet of sea level rise over a longer period of time.
Methane: It’s Not Just in the Arctic
It is important to note that feedbacks due to anthropogenic warming that threaten gas hydrate stability are not only happening in the Arctic. As glaciers everywhere melt, researchers become increasingly concerned that gas could belch into the atmosphere. A study suggests that Antarctic methane could also escape and worsen warming. Other researchers discuss warming in the the most high-elevation regions, like the Third Pole (Tibetan Plateau; Himalayan Mountains), Antarctica, Greenland, the Rockies, the Alps, and so forth. They say that these regions are experiencing significant warming as well and that the higher up you go, the greater the rate of warming. This is not commonly known, but the rate of temperature rise is about 2 times or 3 times or even greater, at higher elevations than at lower elevations, in a phenomena called Elevation Dependent Warming (EDW); clearly analogous to greater warming at higher latitudes.
Methane Sequestration
Methane sequestration has become an important topic as methane levels continue to soar. Methane is removed from the atmosphere (or “sequestered”) when it is absorbed by natural sinks as part of the biological carbon cycle, and using other proposed technologies and geoengineering techniques. Humans are adding +20 million tons per year of methane. As noted above slightly over half of the total emission is due to human activity. This is becoming increasingly necessary science as we move more and more into a methane saturated atmosphere.
The most effective sink of atmospheric methane is the hydroxyl radical in the troposphere, or the lowest portion of Earth’s atmosphere. Methane is normally held in check by the hydroxyl radical (OH), which scrubs nearly all of it out of the atmosphere within a decade. However, OH has been in decline recently. Another natural sink is soil.
New science is suggesting that physical forces that are pushing the methane bubbles up are also pumping nutrient-rich cold waters from the sea bed to the surface, fertilizing phytoplankton blooms that soak up CO2, One study found that surprisingly large CO2 uptake in an area of elevated methane efflux was enhanced relative to surrounding waters, such that the negative radiative forcing effect (cooling) resulting from CO2 uptake overwhelmed the positive radiative forcing effect (warming) supported by methane output.
There are many techniques for methane sequestration being discussed. Most of these are still in the experimental phases with many unknowns. Also, the associated costs are often daunting and not part of the budget of most governments.
Conclusions:
Diverse sources of methane react differently to warming and play a part in the feedback dynamics involved. Both anthropogenic and natural sources of methane combine to present a formidable problem that we as a global species are largely failing to tackle.
On the one hand, the more significant and prevalent anthropogenic methane sources (60%) are already cause for concern presenting significant real-time and real world ramifications for increased greenhouse gas induced warming. While on the other hand, the natural sources (40%), especially wetlands also play a big part with anthropogenic warming induced feedbacks that only amplify their effects. Additionally, the threat looming in the Arctic continues to present a unique and disturbing problem because even though it’s currently a small fraction of the carbon budget at less than 1%, we cannot forget that massive releases have the potential to double greenhouse gas concentrations very abruptly in the Arctic due to polar amplification and other factors to be discussed further here.
One thing is certain, the warmer it gets the closer we get to catastrophe. To continue reading the dramatic, unfolding story of CH4 and learn more about the fascinating and controversial world of methane hydrates, especially in the Arctic, please visit the SW Methane Emergency Wiki >>
Learn more:
- Methane Explained | National Geographic
- Methane Matters | NASA
- Methane: The Other Greenhouse Gas | EDF
- Methane Pollution | David Suzuki Foundation
- Permafrost: Everything You Need to Know | NRDC
- Reducing Methane Pollution | Earth Justice
- Interpreting Contemporary Trends in Atmospheric Methane
__________________________________________________________________
References:
- AMAP, 2015. AMAP Assessment 2015: Methane as an Arctic climate forcer. Retrieved from https://www.amap.no/documents/doc/amap-assessment-2015-methane-as-an-arctic-climate-forcer/1285
- Archer, David (2014) The story of methane in our climate, in five pie charts. Retrieved from http://www.realclimate.org/index.php/archives/2014/09/the-story-of-methane-in-our-climate-in-five-pie-charts/
- EPA (2016). Climate change indicators: climate forcing. Retrieved from https://www.epa.gov/climate-indicators/climate-change-indicators-climate-forcing
- Geophysical Research Letters (2019). Very strong atmospheric methane growth in the four years 2014‐2017. Retrieved from https://agupubs.onlinelibrary.wiley.com/doi/10.1029/2018GB006009
- Geophysical Research Letters (2016). The interaction of climate change and methane hydrates. Retrieved from https://agupubs.onlinelibrary.wiley.com/doi/full/10.1002/2016RG000534
- Harris, William (2019). Fire and ice: the chemistry of methane hydrate. Retrieved from https://science.howstuffworks.com/environmental/green-tech/energy-production/frozen-fuel1.htm
- Hemes et al (2018). A biogeochemical compromise: the high methane cost of sequestering carbon in restored wetlands. Retrieved from https://agupubs.onlinelibrary.wiley.com/doi/10.1029/2018GL077747
- Hymen, Randall (2017). Are methane seeps in the Arctic slowing global warming? Retrieved from https://www.sciencemag.org/news/2017/05/are-methane-seeps-arctic-slowing-global-warming
- IPCC (2013). Climate change 2013: The physical science basis. Retrieved from http://www.ipcc.ch/report/ar5/wg1
- Lauder et al (2013). Offsetting methane emissions…Retrieved from https://www.sciencedirect.com/science/article/pii/S1750583612003064
- NASA (2012). Study finds surprising Arctic methane emissions source. https://www.nasa.gov/topics/earth/features/earth20120422.html
- NASA (2019). A global view of methane. Retrieved from https://earthobservatory.nasa.gov/images/87681/a-global-view-of-methane
- National Geographic (2012). Antarctica methane. Retrieved from https://news.nationalgeographic.com/news/2012/08/120831-antarctica-methane-global-warming-science-environment/
- Nature (2018). Trajectories of the Earth system in the Anthropocene. Retrieved from https://www.pnas.org/content/115/33/8252
- Nature (2017). Enhanced CO2 uptake at a shallow Arctic Ocean seep field overwhelms the positive warming potential of emitted methane. Retrieved from https://www.pnas.org/content/early/2017/05/02/1618926114
- Nisbet et al (2016). Rising atmospheric methane: 2007–2014 growth and isotopic shift. Retrieved from https://agupubs.onlinelibrary.wiley.com/doi/full/10.1002/2016GB005406
- NOAA (2016). The NOAA annual greenhouse gas index. Retrieved from http://www.esrl.noaa.gov/gmd/aggi
- Phys.org (2017). Study finds hydrate gun hypothesis unlikely. Retrieved from https://phys.org/news/2017-08-hydrate-gun-hypothesis.html
- Reviews of Geophysiscs (2017). Methane feedbacks to the global climate System in a warmer world. Retrieved from https://agupubs.onlinelibrary.wiley.com/doi/pdf/10.1002/2017RG000559
- Ruppel et al (2011). Methane hydrates and contemporary climate change. Retrieved from https://www.nature.com/scitable/knowledge/library/methane-hydrates-and-contemporary-climate-change-24314790
__________________________________________________________________
Last Updated: 06/19/2021